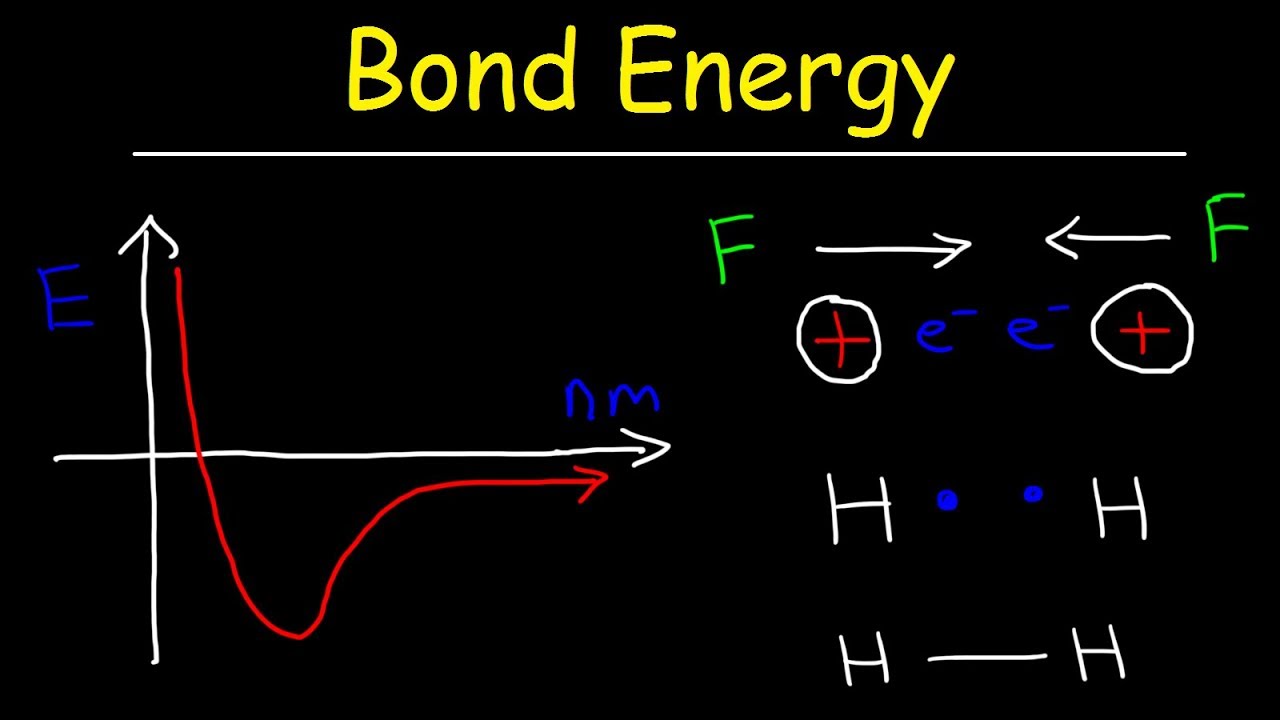
Bond Energy Diagram Bond Enthalpy (bond Energy)
Covalent bond energy and length Solved * bond energy and enthalpy a table 13.6 average bond Bond energy
Bond Energies - Chemwiki
Ionic bond bonding potential interaction internuclear electrostatic atoms ions released formed molecular Openstax: general chemistry Bond atoms chemistry energy bonding theory covalent valence first distance length two interaction system general graph hydrogen diagram shown between
Valence bond theory
Atoms potential hydrogen bond covalent changes separation electron ck structuresBond chemistry energy bonding theory covalent valence distance atoms length two interaction system shown graph hydrogen diagram between curve internuclear Binding nuclear energies mass britannica physicsBond energy: definition & equation.
Bond bonding energy structure chapter stability ppt powerpoint presentationLesson video: bond energy Energy bond exothermic formation diagram chemical bonds released when broken forming change endothermic negative enthalpy releases process always its reactionsEnergies bonding calculate kj calculations.

Lewis electron dot structures
Bond energies energy chemwiki enthalpyBond energy potential bonding covalent atoms lengths energies two chemical chemistry molecule breaking when distance why bonds curve hydrogen formation Energy and covalent bond formationBond length energy graph distance bonds.
Bond energies5.2: valence bond theory Solved table 8.6 average bond dissociation energies and bondEnergy bond forming releases chemical bonds formation enthalpy exothermic negative process always change its.

Energy bond atoms when released between definition break science two needed saved particular forms
Bond energy calculationsBond enthalpy (bond energy) Bond energy length chemistry forces repulsion attractionBond energy & bond length, forces of attraction & repulsion.
Energy potential bond atoms covalent formation bonds two distance hydrogen chemistry graph separation electron bonding changes function shows water theirBond energy covalent length Chemistry bond energy potential chemical covalent bonding two electron versus atoms hydrogen diagram valence theory ionic water lewis distance betweenBond energy definition breaking water oxygen molecules hydrogen form equation apart study.

Bond energy enthalpy hydrogen chemistry energies bonds bonding ethene kj mol gas broken formed table values chemical reaction data h2o
How to find bond energy from a graphChapter 4.1: ionic bonding Bond sciencenotesTable of bond energies.
Bond energy strength 2021 helmenstine anne entry updated january posted mayEnergy and covalent bond formation Bond energy calculations & enthalpy change problems, basic introductionBond lengths and energies.

Bond energy
Nuclear binding energyEnergy and covalent bond formation Exothermic and endothermic reactions + bond energies diagramBond enthalpy energy change chemistry calculations problems basic.
Chemistry archivesBond energy Bond energyBond energy and strength.
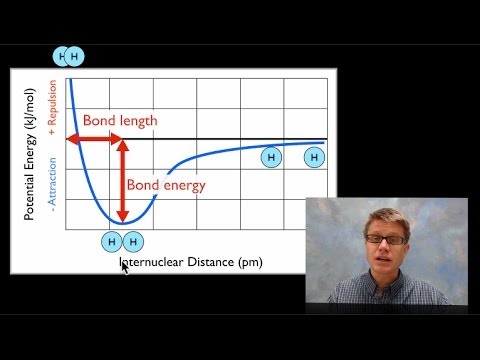
Energy bond chemical metabolism released formed when amount break ppt powerpoint presentation atoms
Bond energy and strengthEnergy potential bond atoms formation two distance hydrogen chemistry graph covalent separation electron changes function shows their dot structures lewis Bond length and bond energyBond energy and strength.
D7.1 bond length and bond energy – chemistry 109 fall 2021 .